Nickel cadmium battery contributors and attributions rechargeable batteries also known as secondary cells are batteries that potentially consist of reversible cell reactions that allow them to recharge or regain their cell potential through the work done by passing currents of electricity.
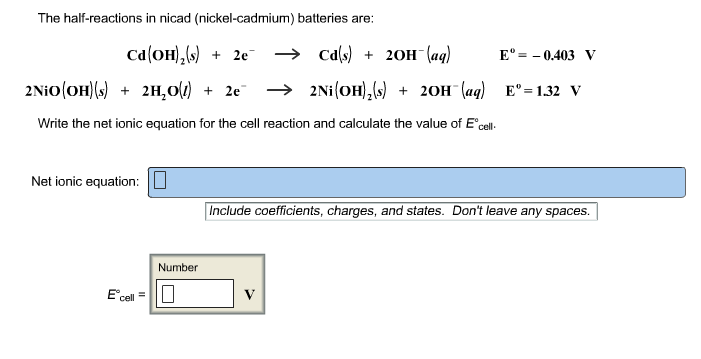
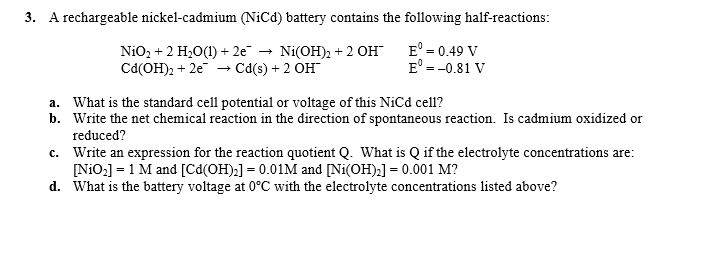
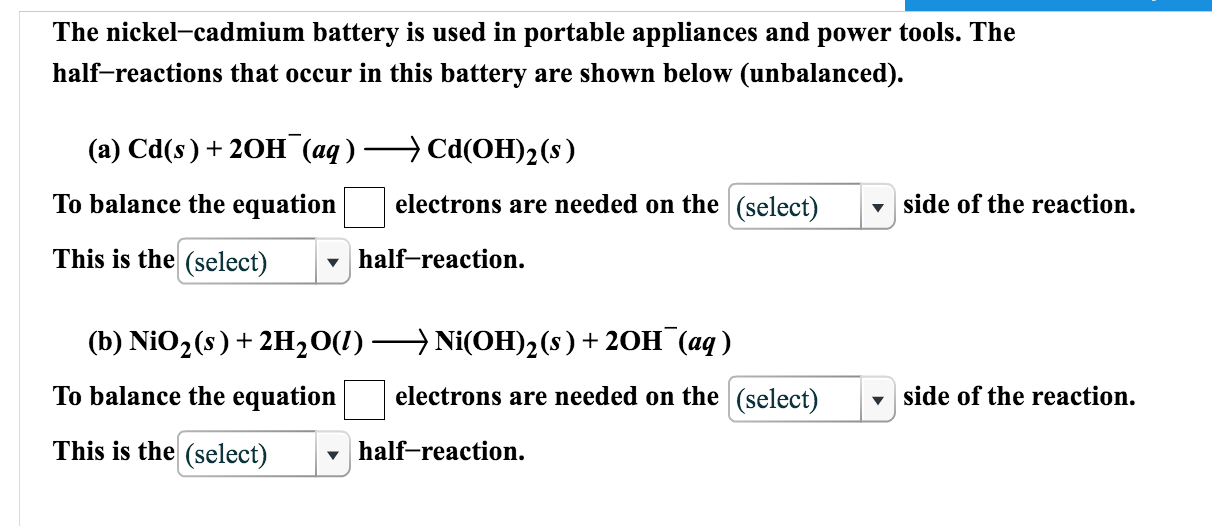
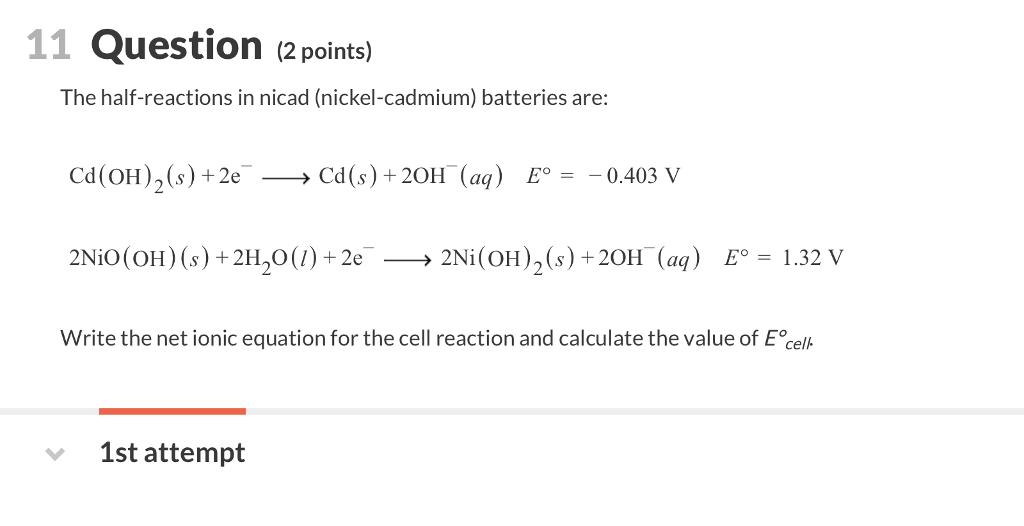
Nickel cadmium battery reaction equation.
The electrolyte is a solution of potassium hydroxide koh with a small addition of lithium hydrate which increases the capacity and life of the battery.
The chemical conversion is reverted when a discharged battery is charged again.
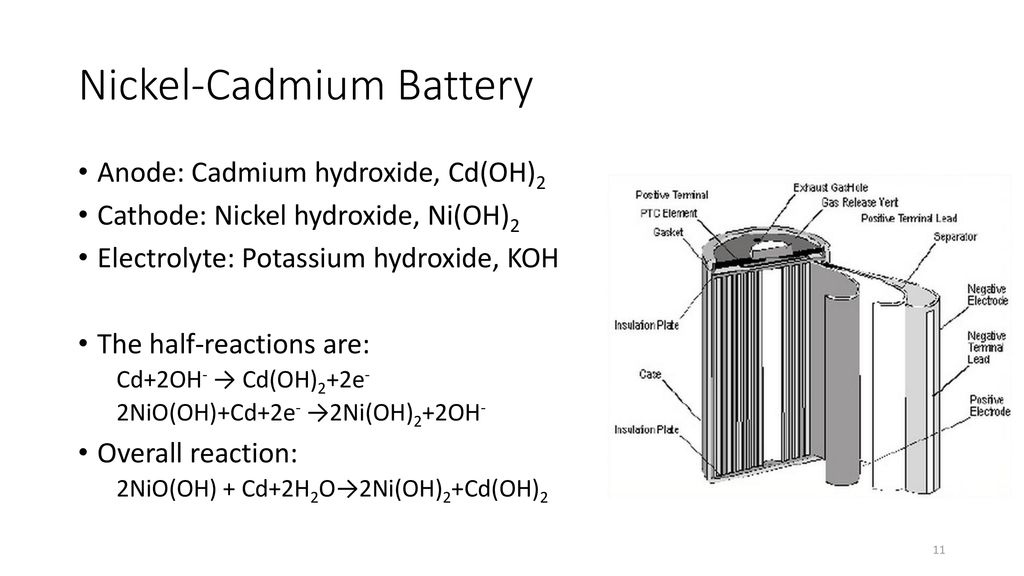
A nickel cadmium cell has two plates.
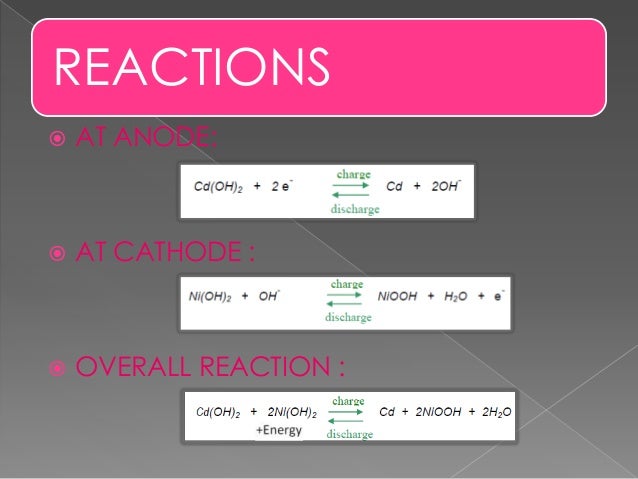
The reactions at the nickel oxide electrode are.
The nickel cadmium battery system still uses the same positive electrode as the nickel iron one while the negative electrode is cadmium.
The abbreviation ni cd is derived from the chemical symbols of nickel.
The nickel cadmium battery ni cd battery or nicad battery is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes.
The chemical reaction in the nickle cadmium battery or cell is perfectly reversible.
Nickel cadmium batteries are used in various developmental electric vehicles.
In the case of the nickel cadmium battery the cadmium electrode has two important features.
When fully charged the positive plate is ni oh 2 and negative plate is cd oh 2 while discharging the positive plate converts into ni oh 3 and the negative plate is converted into pure cd.
They operate over a wide temperature range give approximately 2000 cycles and can be charged in less than 1 h.
Nickel cadmium batteries at the 1 2h rate yield twice the energy density of lead acid batteries i e.
The maximum cell voltage during charge is 1 3 v and the average cell voltage is 1 2 v.
The active material of the positive plate anode is ni oh 4 and the negative plate cathode is of cadmium cd when fully charged.